Duration of Treatment
50000 to 85000 USD
Days of Stay
30 Days
Anesthesia
No
Cost
50000 to 85000 USD
How Much Does CAR T Cell Therapy Cost in India?
Looking for CAR T cell therapy cost in India in different cities like Delhi, Mumbai, Chennai, Kolkata, and Bangalore? Here, we answer the question and explain how to choose the best treatment centers in India for better results. Still, have questions? We have also shortlisted the list of cancer centers in India and doctors based on Hospital accreditations, experience & qualification of surgeons, success rates of procedures, and patient testimonials.
- CART cell therapy cost in India: Starting from US$ 50,000 and US$ 85,000
- Hotel Cost Near Hospital - starting from 18 to 50 USD ( as per hotel services)
- Food Cost - starting from 20 to 30 USD (per day )
- Miscellaneous cost - 20 USD (per day)
To make an appointment, learn more about it, read the below information, or call / WhatsApp/ Viber - our experts to answer at +91 9582708782. Still, have questions?
Cost Variation by City:
- Delhi: Top hospitals like AIIMS and Fortis generally charge in the higher range of the pricing spectrum.
- Mumbai: Leading hospitals such as Tata Memorial and HCG are known for offering high-quality CAR T-cell therapy, with costs typically in the mid to high range.
- Chennai: Hospitals like Apollo Cancer Centre and Sri Ramachandra Medical Centre offer competitive pricing, with costs typically lower than in Delhi or Mumbai.
- Kolkata: Well-regarded hospitals like Tata Medical Center offer affordable treatment options, with pricing around the lower to mid-range.
- Bangalore: Manipal Hospitals and Fortis offer CAR T-cell therapy in the mid to high range.
How to Choose the Best Treatment Centers in India:
When selecting a treatment center for CAR T-cell therapy, consider the following factors:
- Hospital Accreditation: Look for NABH, NABL, or JCI-accredited hospitals, which ensure quality care and standards.
- Experience & Qualifications of Surgeons: Experienced oncologists and hematologists specializing in CAR T-cell therapy are essential for successful outcomes.
- Success Rates: Research success rates for CAR T-cell therapy, especially for the specific cancer type you're being treated for.
- Patient Testimonials: Look for positive patient reviews and testimonials, particularly from those who have undergone CAR T-cell therapy at the hospital.
- Post-Treatment Care: Consider the hospital’s post-treatment care protocols, as CAR T-cell therapy can require extensive follow-up.
Contact Information:
If you’re interested in scheduling a consultation, you can contact experts or receive more detailed information by calling or messaging:
- Phone/WhatsApp/Viber: +91 9582708782
Summary:
The cost of CAR T-cell therapy in India is considerably lower than in many Western countries, with a starting price of around US$ 50,000, depending on the city and hospital. In addition to the therapy itself, other expenses such as accommodation, food, and miscellaneous costs should be factored in. By choosing an accredited hospital with experienced doctors, you can improve your chances of successful treatment.
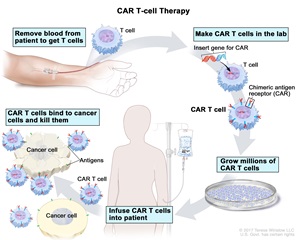
What is CAR T Cell Therapy?
CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy) is an advanced form of immunotherapy used primarily to treat certain types of cancer, particularly blood cancers such as leukemia, lymphoma, and multiple myeloma. It involves reprogramming a patient’s own immune cells to target and kill cancer cells.
CAR T-cell therapy comes in various types, categorized based on the specific antigens they target or the technologies used to improve their effectiveness. Here are the primary types of CAR T-cell therapies:
1. Antigen-Specific CAR T-Cell Therapies
These therapies are classified by the specific protein (antigen) they target on cancer cells:
-
CD19 CAR T-cell Therapy:
- Targets CD19, a protein commonly found on B cells.
- Effective for:
- Acute Lymphoblastic Leukemia (ALL)
- Diffuse Large B-Cell Lymphoma (DLBCL)
- Mantle Cell Lymphoma
- Examples: Kymriah (tisagenlecleucel), Yescarta (axicabtagene ciloleucel), Breyanzi (lisocabtagene maraleucel).
-
BCMA CAR T-cell Therapy:
- Targets BCMA (B-cell maturation antigen), found on plasma cells.
- Effective for:
- Multiple Myeloma
- Examples: Abecma (idecabtagene vicleucel), Carvykti (ciltacabtagene autoleucel).
-
CD22 CAR T-cell Therapy:
- Targets CD22, another protein found on B cells.
- Primarily used in research or for cases where CD19 therapy is less effective.
-
Dual-Antigen CAR T-cell Therapy:
- Targets two antigens (e.g., CD19 and CD22) simultaneously.
- Designed to overcome resistance by ensuring cancer cells cannot evade therapy by losing a single antigen.
2. Generational CAR T-Cell Therapies
CAR T-cell therapy has evolved over time, with improvements in design:
-
First-Generation CARs:
- Basic design with limited effectiveness.
- Rarely used today.
-
Second-Generation CARs:
- Incorporate one costimulatory domain (e.g., CD28 or 4-1BB) to enhance T-cell activation and survival.
- Most FDA-approved therapies fall into this category.
-
Third-Generation CARs:
- Combine multiple costimulatory domains for greater potency.
- Primarily used in research settings.
-
Fourth-Generation CARs (TRUCKs):
- "T-cells Redirected for Universal Cytokine Killing."
- Engineered to deliver cytokines to enhance the immune response at the tumor site.
-
Fifth-Generation CARs:
- Include intracellular signaling pathways to improve efficacy and manage resistance.
3. Universal or Off-the-Shelf CAR T-Cell Therapies
- Allogeneic CAR T-Cell Therapy:
- Uses T cells from a healthy donor rather than the patient’s own cells.
- Reduces wait times and makes therapy more accessible.
- Example: Research in therapies like AlloCAR T.
4. Tumor Microenvironment-Targeting CAR T-Cell Therapies
- Designed to address barriers in the tumor environment, such as immune suppression or poor T-cell infiltration.
- Still largely in experimental stages.
5. Solid Tumor CAR T-Cell Therapies
- Target antigens like HER2, EGFR, or GD2, which are expressed in solid tumors.
- Development is more challenging due to antigen heterogeneity and the immunosuppressive nature of solid tumors.
These types of CAR T-cell therapies represent a rapidly advancing field, with ongoing research aimed at making them safer, more effective, and applicable to a wider range of cancers.
Is CAR-T cell therapy Available in India?
Yes, CAR T-cell therapy is available in India. In April 2024, India celebrated the launch of its first homegrown CAR T-cell therapy, NexCAR19™ (actalycabtagene autoleucel), developed through a collaboration between the Indian Institute of Technology, Bombay, Tata Memorial Hospital, and ImmunoACT.
This therapy has been approved for the treatment of relapsed or refractory B-cell lymphomas and leukemia in patients aged 15 years and above.
car-t cell therapy hospitals in India : Several leading hospitals in India now offer CAR T-cell therapy:
-
Apollo Cancer Centres: As the first private hospital group in India to launch a CAR T-cell program, Apollo provides access to NexCAR19™ for treating B-cell lymphomas and B-acute lymphoblastic leukemia.
-
Max Healthcare: Located in Delhi, Max Healthcare offers CAR T-cell therapy for various blood cancers, including acute lymphoblastic leukemia and diffuse large B-cell lymphoma.
-
Artemis Hospital: Gurugram, India, offers advanced cancer treatments, including CAR T-cell therapy. This therapy involves modifying a patient's T-cells to enhance their ability to combat cancer. Artemis Hospitals has successfully treated patients with aggressive forms of lymphoma using CAR T-cell therapy. For instance, Ms. Linda Becht from the Netherlands underwent successful treatment for an aggressive form of lymphoma at Artemis Hospitals.
For more detailed information or to determine eligibility for CAR T-cell therapy at Artemis Hospitals, it is advisable to contact their oncology department directly.
The cost of CAR T-cell therapy in India ranges between US$50,000 and US$85,000, which is significantly more affordable compared to prices in countries like the United States, where costs can exceed $400,000.
This development positions India as a key player in providing advanced and cost-effective CAR T-cell therapies, making it accessible to a broader patient population.
n India, the cost of CAR T-cell therapy ranges from approximately ₹30–40 lakh (3–4 million INR) as of April 2024. This cost is significantly lower compared to the treatment in other countries like the United States, where it can exceed $400,000.
Efforts to Reduce Costs
- There is a concerted effort to further reduce the cost in India to ₹10–20 lakh (1–2 million INR) in the future.
- The lower cost in India is partly due to more affordable labor and localized manufacturing.
Insurance Coverage
- Some insurance companies in India may cover CAR T-cell therapy for eligible patients, but not all policies include this treatment. It is advisable to check with individual insurers.
NexCAR19
- NexCAR19 is a CAR T-cell therapy developed by ImmunoACT, a collaboration involving the Indian Institute of Technology Bombay, Tata Memorial Hospital, and others.
- It is recognized as one of the most affordable CAR T-cell therapies globally.
- ImmunoACT manufactures CAR T cells at its facility in Mumbai, ensuring localized production and lower costs.
Why is CAR T-cell Therapy Cheaper in India?
- Localized Manufacturing: Facilities in Mumbai produce the therapy domestically, avoiding international transportation and import costs.
- Lower Labor Costs: India's healthcare and research sectors benefit from lower operational expenses.
- Government and Research Support: Collaborative efforts with institutions like IIT Bombay and Tata Memorial Hospital help subsidize development costs.
Conclusion
India is emerging as a global hub for CAR T-cell therapy, offering advanced treatment options at significantly reduced costs while maintaining high standards of care.
Who is a candidate for CAR T-cell therapy?
Candidates for CAR T-cell therapy are typically patients with certain types of cancers who meet specific criteria. This therapy is most often used for people who have not responded to standard treatments or whose cancer has returned after remission. Here's an overview of who might be eligible:
1. Types of Cancers Treated
CAR T-cell therapy is primarily approved for the following cancers:
- Blood Cancers:
- Acute Lymphoblastic Leukemia (ALL):
- Particularly in children and young adults.
- Candidates often have relapsed or refractory (R/R) disease.
- B-Cell Non-Hodgkin Lymphomas:
- Diffuse Large B-Cell Lymphoma (DLBCL)
- Mantle Cell Lymphoma (MCL)
- Follicular Lymphoma (FL)
- Multiple Myeloma:
- Targeting BCMA antigen in relapsed/refractory cases.
- Acute Lymphoblastic Leukemia (ALL):
- Other Emerging Indications:
- Research trials are exploring CAR T-cell therapy for solid tumors (e.g., glioblastoma, lung cancer) and additional blood cancers.
2. Criteria for Eligibility
Patients must generally meet these conditions to qualify:
- Relapsed or Refractory Cancer:
- Cancer that has not responded to two or more lines of conventional treatment (e.g., chemotherapy, radiation, targeted therapy).
- Adequate Organ Function:
- Sufficient heart, liver, kidney, and lung function to handle the therapy and potential side effects.
- Good Performance Status:
- Patients should have a reasonable level of physical fitness (e.g., ECOG performance status of 0-2).
- No Active Infections:
- To reduce the risk of complications during therapy.
- Appropriate Age:
- Age limits may vary based on cancer type, clinical trial criteria, or specific therapy protocols (e.g., some therapies are approved for pediatric patients with ALL).
3. Exclusions
Patients may not qualify if they have:
- Active or uncontrolled infections.
- Severe organ dysfunction or poor overall health.
- Autoimmune diseases that could be exacerbated by therapy.
- Recent history of other aggressive cancers.
4. Special Considerations
- Clinical Trials:
- Patients with cancers not yet approved for CAR T-cell therapy (e.g., solid tumors) may participate in clinical trials if they meet the inclusion criteria.
- Insurance and Cost:
- Availability and eligibility can also depend on financial or insurance coverage for the treatment.
5. Personalized Evaluation
Each candidate is thoroughly evaluated by an oncology and immunotherapy team to ensure suitability, balancing potential benefits with risks. This includes diagnostic tests, imaging, and a review of medical history.
What is the Risk for CART cell therapy?
CAR T-cell therapy, while highly effective for certain cancers, carries significant risks due to its potent immune-modulating effects. These risks stem from the therapy's mechanism of action, where engineered T cells aggressively attack cancer cells but can also affect normal tissues or trigger systemic immune responses. Below are the primary risks and side effects associated with CAR T-cell therapy:
1. Cytokine Release Syndrome (CRS)
- What It Is: A systemic inflammatory response caused by the rapid activation and proliferation of CAR T cells, leading to the release of large amounts of cytokines.
- Symptoms:
- High fever
- Low blood pressure
- Difficulty breathing
- Rapid heart rate
- Organ dysfunction (e.g., kidneys, liver)
- Severity:
- Ranges from mild to life-threatening (grade 1–4).
- Management:
- Anti-inflammatory drugs like tocilizumab (IL-6 receptor antagonist) or corticosteroids.
2. Neurological Toxicity (Immune Effector Cell-Associated Neurotoxicity Syndrome, ICANS)
- What It Is: Neurotoxicity caused by the immune response, often accompanying or following CRS.
- Symptoms:
- Confusion or delirium
- Seizures
- Difficulty speaking or slurred speech
- Loss of consciousness
- Management:
- Supportive care and, in severe cases, corticosteroids.
3. Prolonged Low Blood Cell Counts (Cytopenias)
- What It Is: Suppression of normal blood cell production in the bone marrow.
- Risks:
- Anemia (low red blood cells)
- Increased risk of infections (low white blood cells)
- Bleeding tendencies (low platelets)
- Management:
- Blood transfusions or growth factors (e.g., G-CSF).
4. Infections
- What It Is: Increased susceptibility to infections due to immune suppression.
- Causes:
- Initial lymphodepleting chemotherapy before CAR T infusion.
- Prolonged depletion of B cells (hypogammaglobulinemia).
- Management:
- Antibiotics, antivirals, or antifungals.
- Immunoglobulin replacement therapy.
5. Tumor Lysis Syndrome (TLS)
- What It Is: A condition caused by the rapid breakdown of cancer cells, releasing their contents into the bloodstream.
- Risks:
- Kidney damage
- Electrolyte imbalances (e.g., high potassium, uric acid, and phosphate levels)
- Management:
- Hydration, medications like allopurinol, and monitoring electrolyte levels.
6. On-Target, Off-Tumor Toxicity
- What It Is: Damage to normal cells expressing the same antigen as cancer cells.
- Risks:
- Varies depending on the targeted antigen (e.g., CD19 can also affect normal B cells).
7. Graft Versus Host Disease (GVHD) (in allogeneic CAR T-cell therapies):
- What It Is: Immune reaction where donor T cells attack the recipient's tissues.
- Management:
- Immunosuppressive therapy.
8. Long-Term Risks
- Relapse or Resistance: Cancer may return or become resistant to CAR T cells.
- Secondary Cancers: Rare cases of therapy-induced malignancies.
- Long-Term Immunosuppression: Persistent depletion of normal immune cells.
9. Psychological and Emotional Impact
- The intensive nature of treatment and side effects can lead to emotional and mental health challenges, including anxiety and depression.
Mitigation Strategies
- Close Monitoring: Patients are monitored closely in specialized centers for early detection and management of side effects.
- Multidisciplinary Care: A team of oncologists, immunologists, and other specialists ensures comprehensive care.
- Patient Selection: Only patients with adequate organ function and overall health are considered for therapy.
While the risks of CAR T-cell therapy are substantial, advancements in management and early detection have significantly improved patient outcomes.
What will be the preparation before car t cell therapy?
Preparation for CAR T-cell therapy is a detailed process designed to maximize its effectiveness while minimizing potential risks. Patients undergo several steps, including evaluations, pre-treatment procedures, and supportive care planning. Here's what preparation typically involves:
1. Initial Evaluation
- Medical History and Physical Exam:
- A thorough review of the patient’s medical history, current medications, and overall health.
- Cancer Assessment:
- Tests to confirm the type, stage, and status of the cancer.
- Imaging studies (CT, PET, or MRI scans).
- Lab Tests:
- Blood work to evaluate organ function (kidney, liver, heart), infection risk, and overall health.
- Bone Marrow Biopsy:
- For patients with blood cancers, to assess the extent of disease.
2. Eligibility Assessment
- Performance Status:
- Determination of the patient’s physical ability to tolerate the therapy (e.g., ECOG or Karnofsky Performance Status).
- Organ Function:
- Heart: Echocardiogram or MUGA scan.
- Lungs: Pulmonary function tests.
- Liver and Kidney: Comprehensive metabolic panel.
- Infection Screening:
- Testing for infections such as HIV, hepatitis, and tuberculosis to ensure safety during therapy.
3. Collection of T Cells (Leukapheresis)
- Leukapheresis Procedure:
- T cells are collected from the patient’s blood using a machine that separates and collects white blood cells.
- The process takes several hours and may require multiple sessions.
- Post-Collection Care:
- Patients are monitored for side effects, such as fatigue or low calcium levels.
4. Waiting Period (Manufacturing Phase)
- CAR T-Cell Production:
- The collected T cells are genetically engineered in a laboratory to produce chimeric antigen receptors (CARs) that target cancer cells.
- This process typically takes 2–4 weeks.
- Bridging Therapy (if needed):
- If the cancer is progressing rapidly, patients may receive interim treatments (e.g., chemotherapy, radiation) to control disease until the CAR T cells are ready.
5. Preconditioning Chemotherapy (Lymphodepletion)
- Purpose:
- Low-dose chemotherapy is given to suppress the immune system, creating space for the infused CAR T cells to expand and work effectively.
- Timing:
- Administered a few days before the CAR T-cell infusion.
- Common Drugs Used:
- Cyclophosphamide and fludarabine.
6. Hospital Admission
- Hospitalization:
- Many patients are admitted to a specialized cancer center for monitoring during and after the CAR T-cell infusion.
- In some cases, outpatient therapy is possible, but close proximity to the hospital is required.
7. Supportive Care Planning
- Infection Prevention:
- Prophylactic antibiotics, antivirals, or antifungals may be prescribed.
- Emergency Plan:
- Detailed protocols for managing potential side effects like cytokine release syndrome (CRS) or neurotoxicity.
- Caregiver Support:
- Patients need a caregiver or family member to assist during recovery.
- Psychological Preparation:
- Counseling or support groups may help address emotional and mental health concerns.
8. Consent and Education
- Informed Consent:
- Patients are educated about the risks, benefits, and potential outcomes of CAR T-cell therapy.
- Educational Sessions:
- Detailed explanations about the therapy process, side effects, and post-treatment expectations.
9. Nutritional and Physical Preparation
- Healthy Diet:
- Maintaining good nutrition to support immune function and recovery.
- Physical Activity:
- Gentle exercise to improve physical fitness before treatment.
Summary Timeline
- Weeks 1–2: Initial evaluation and eligibility determination.
- Week 3: Leukapheresis (T-cell collection).
- Weeks 4–8: CAR T-cell production; bridging therapy if needed.
- Final Week: Preconditioning chemotherapy and admission for infusion.
This detailed preparation helps ensure patients are in the best possible condition to receive CAR T-cell therapy and manage its potential side effects effectively.
what will be the procedure CART cell therapy?
The procedure for CAR T-cell therapy involves several steps, from the collection of T cells to their genetic modification, infusion, and post-treatment monitoring. Here's an overview of the process:
1. T-Cell Collection (Leukapheresis)
- Purpose: To collect T cells from the patient's blood.
- Procedure:
- The patient is connected to a machine via an IV line or central catheter.
- Blood is drawn, and the machine separates white blood cells (including T cells) from the rest of the blood.
- The remaining blood components are returned to the patient.
- Duration: Takes about 2–4 hours and is generally done in an outpatient setting.
2. Genetic Modification (CAR T-Cell Manufacturing)
- Purpose: To engineer the patient’s T cells to better recognize and attack cancer cells.
- Procedure:
- The collected T cells are sent to a specialized laboratory.
- The cells are genetically modified using a viral vector to add a chimeric antigen receptor (CAR), enabling them to target a specific protein (antigen) on cancer cells.
- The modified T cells are then grown and expanded in the lab to produce millions of CAR T cells.
- Duration: This step takes 2–4 weeks.
3. Preconditioning Chemotherapy (Lymphodepletion)
- Purpose: To suppress the patient’s immune system and create a favorable environment for the infused CAR T cells to work effectively.
- Procedure:
- Low-dose chemotherapy is administered over 2–3 days before the CAR T-cell infusion.
- Common drugs: Cyclophosphamide and Fludarabine.
- Monitoring: Patients are closely monitored for side effects like fatigue or low blood counts.
4. CAR T-Cell Infusion
- Purpose: To reintroduce the engineered CAR T cells into the patient’s body to attack cancer cells.
- Procedure:
- CAR T cells are thawed and infused intravenously, similar to a blood transfusion.
- The infusion usually takes 30–60 minutes but may vary depending on the dose.
- Hospital Setting:
- This step is performed in a specialized cancer center.
- Patients are monitored closely during the infusion for immediate side effects.
5. Post-Infusion Monitoring
- Purpose: To manage potential side effects and monitor the effectiveness of the therapy.
- Steps:
- In-Hospital Monitoring:
- Patients are typically observed for 7–14 days for acute complications like Cytokine Release Syndrome (CRS) or neurotoxicity.
- Post-Discharge Monitoring:
- Regular follow-ups for 4–8 weeks to monitor recovery and response to therapy.
- Long-Term Monitoring:
- Periodic check-ups for up to a year or more to assess the treatment’s success and detect any delayed side effects.
- In-Hospital Monitoring:
6. Management of Side Effects
- Cytokine Release Syndrome (CRS):
- Symptoms: Fever, low blood pressure, and breathing difficulties.
- Treated with medications like tocilizumab and corticosteroids.
- Neurotoxicity (ICANS):
- Symptoms: Confusion, seizures, or speech issues.
- Treated with supportive care and corticosteroids.
7. Follow-Up and Support
- Efficacy Evaluation:
- Imaging scans (CT, PET) and blood tests are performed to determine the cancer’s response to therapy.
- Rehabilitation:
- Physical and emotional support for recovery.
- Immunological Monitoring:
- Monitoring for prolonged immune suppression or relapse.
- Secondary Treatments:
- Additional interventions may be required if the cancer doesn’t respond fully.
Summary Timeline
- T-Cell Collection: 1 day.
- T-Cell Engineering: 2–4 weeks.
- Preconditioning Chemotherapy: 2–3 days.
- CAR T-Cell Infusion: 1 day.
- Post-Treatment Monitoring: Weeks to months.
This structured approach ensures safety and maximizes the effectiveness of CAR T-cell therapy for patients with eligible cancers.
What will be the post-care for CAR T Cell therapy?
Post-care for CAR T-cell therapy is critical to monitor for side effects, evaluate the therapy’s effectiveness, and ensure a smooth recovery. The post-care period involves close observation, regular follow-ups, and supportive care to address potential complications and long-term effects. Here's a detailed breakdown of post-care:
1. Immediate Post-Treatment Monitoring
- Hospital Stay:
- Most patients are monitored in the hospital for 1–2 weeks after the CAR T-cell infusion.
- Vital signs and symptoms are observed for immediate side effects, especially Cytokine Release Syndrome (CRS) and neurotoxicity (ICANS).
- Treatments like tocilizumab (IL-6 inhibitor) or corticosteroids may be administered as needed.
- Isolation Precautions:
- To prevent infections due to temporary immune suppression, patients are often kept in sterile environments.
2. Short-Term Care (First 4–8 Weeks)
- Frequent Medical Visits:
- Regular follow-ups with the oncology team to monitor recovery, manage side effects, and evaluate the therapy’s effectiveness.
- Blood tests to assess organ function, immune cell counts, and signs of relapse.
- Infection Prevention:
- Preventive antibiotics, antivirals, or antifungals may be prescribed.
- Vaccination schedules are deferred or carefully planned to avoid live vaccines during immune suppression.
- Symptom Management:
- Addressing fatigue, nausea, pain, or other lingering effects from chemotherapy or the infusion process.
- Emotional and Psychological Support:
- Counseling or support groups to help patients cope with the emotional challenges of cancer treatment.
3. Long-Term Follow-Up (Months to Years)
- Efficacy Evaluation:
- Imaging (CT, PET scans) and bone marrow biopsies to confirm cancer remission or response to treatment.
- Regular blood tests to monitor for cancer recurrence.
- Monitoring for Late-Onset Side Effects:
- Persistent cytopenias (low blood cell counts).
- Delayed neurological symptoms.
- Prolonged immune suppression leading to infections.
- B-Cell Aplasia (if CAR T cells target CD19):
- Patients may need immunoglobulin replacement therapy to prevent infections.
4. Rehabilitation and Quality of Life
- Physical Rehabilitation:
- Physical therapy to regain strength and stamina after prolonged hospitalization or treatment.
- Nutritional Support:
- A balanced diet to support recovery and immune function.
- Mental Health Support:
- Addressing anxiety, depression, or post-traumatic stress related to cancer and its treatment.
5. Secondary Treatments or Maintenance
- Treatment for Residual Cancer:
- In cases where cancer persists, additional therapies may be considered (e.g., radiation, chemotherapy, or clinical trials).
- Management of Relapse:
- Patients are monitored closely for signs of cancer recurrence and may undergo another round of CAR T-cell therapy or alternative treatments.
6. Education for Patients and Caregivers
- Symptom Awareness:
- Patients and caregivers are trained to recognize early signs of complications, such as fever, confusion, or shortness of breath.
- Emergency Plan:
- Clear instructions on when and how to seek medical help in case of severe symptoms.
- Lifestyle Adjustments:
- Avoiding exposure to infections (e.g., crowded places).
- Modifications to work or daily activities based on recovery progress.
7. Re-Vaccination
- Vaccination Schedule:
- Following immune recovery, patients may need re-vaccination, particularly for diseases like measles, mumps, rubella, and pneumococcus.
Potential Long-Term Risks to Monitor
- Cancer Relapse or Resistance:
- Periodic testing to check for recurrence or resistance to CAR T cells.
- Secondary Malignancies:
- Rare cases of treatment-related cancers.
- Chronic Graft-Versus-Host Disease (in allogeneic CAR T therapy):
- Long-term immunosuppressive management if this occurs.
8. Financial and Logistical Support
- Insurance and Cost Management:
- Assistance with claims or financial aid for follow-up care and medications.
- Travel Logistics:
- Patients treated far from home may need to stay near the treatment center for follow-ups during the first few months.
Summary
Post-care for CAR T-cell therapy is a long-term process aimed at:
- Managing acute and late-onset side effects.
- Monitoring the therapy’s success in treating cancer.
- Providing physical, emotional, and logistical support for recovery.
Patients and caregivers play an essential role in ensuring adherence to follow-up schedules and promptly reporting any concerning symptoms.
What will be the success rate for CAR T-cell therapy?
The success rate of CAR T-cell therapy varies depending on several factors, including the type of cancer being treated, the patient’s overall health, the specific CAR T-cell product used, and how well the patient responds to the therapy. However, CAR T-cell therapy has shown impressive results, particularly for certain blood cancers. Below are general success rates and factors that influence outcomes:
Success Rates in Blood Cancers (Most Common Indications)
-
Acute Lymphoblastic Leukemia (ALL)
- Children and Young Adults: For B-cell ALL, especially in pediatric and young adult patients, CAR T-cell therapy has shown a high response rate.
- Complete Remission: Around 80–90% of children and young adults with relapsed or refractory B-ALL achieve complete remission (CR) after CAR T-cell therapy.
- Long-Term Remission: However, about 50–60% of these patients may experience long-term remission, with the remainder relapsing or experiencing recurrence within 1–2 years.
- Children and Young Adults: For B-cell ALL, especially in pediatric and young adult patients, CAR T-cell therapy has shown a high response rate.
-
Diffuse Large B-Cell Lymphoma (DLBCL)
- In patients with relapsed or refractory DLBCL, CAR T-cell therapy has demonstrated a response rate of approximately:
- Overall Response Rate: Around 50–60% of patients show a positive response.
- Complete Remission: Roughly 30–40% of patients achieve complete remission.
- However, outcomes can be affected by factors such as age, prior treatments, and the specific subtype of lymphoma.
- In patients with relapsed or refractory DLBCL, CAR T-cell therapy has demonstrated a response rate of approximately:
-
Multiple Myeloma
- CAR T-cell therapy targeting BCMA (B-cell maturation antigen) in multiple myeloma has shown promising results:
- Response Rate: Around 70–80% of patients with relapsed/refractory multiple myeloma experience a positive response.
- Complete Remission: Approximately 30–40% may achieve complete remission, though relapses are common.
- CAR T-cell therapy targeting BCMA (B-cell maturation antigen) in multiple myeloma has shown promising results:
-
Chronic Lymphocytic Leukemia (CLL)
- For CLL, especially when it relapses or becomes resistant to other treatments, CAR T-cell therapy has a response rate of 50–70%, with a complete remission rate of around 30–40%.
Success Rates in Solid Tumors
- Solid tumors (e.g., lung cancer, breast cancer, glioblastoma) are more challenging to treat with CAR T-cell therapy.
- Response Rates: Early clinical trials have shown limited success, with response rates generally below 30%.
- Challenges: Solid tumors present difficulties like immune suppression by the tumor microenvironment, antigen heterogeneity, and physical barriers (e.g., the blood-brain barrier).
- Ongoing research is focused on improving CAR T-cell effectiveness for solid tumors, and success rates may improve as new therapies are developed.
Factors Affecting Success Rates
- Type of Cancer: Blood cancers like leukemia and lymphoma tend to have better outcomes with CAR T-cell therapy than solid tumors.
- Disease Stage:
- Patients with early-stage cancer or those who achieve a deep remission before CAR T-cell therapy generally have better outcomes.
- Relapsed or refractory cases may have lower success rates, especially if the cancer has developed resistance to previous treatments.
- Patient Characteristics:
- Age: Younger patients, especially children and young adults, tend to have higher response rates.
- Health and Performance Status: Patients in good overall health, with well-functioning organs and good performance status, are more likely to have a positive outcome.
- CAR T-Cell Product: Different CAR T-cell products may vary in efficacy, with newer therapies and customized treatments showing higher success in some cases.
- Manufacturing Success: The quality of T-cell manufacturing (e.g., expansion and persistence of the engineered cells) can impact the success of the therapy.
- Side Effects: Severe side effects (e.g., cytokine release syndrome, neurotoxicity) can negatively impact treatment success, either temporarily or permanently.
Relapse and Long-Term Outcomes
-
Relapse:
- Even after initial remission, some patients may relapse within months or years of CAR T-cell therapy. The relapse rate is generally higher in patients with more aggressive or refractory cancers.
- New treatments or second-line CAR T-cell therapies are being explored for these cases.
-
Long-Term Success:
- Long-term survival rates have improved, especially for patients with blood cancers like ALL, DLBCL, and multiple myeloma, but the success rate is not guaranteed for every patient.
Overall Summary of Success Rates
- Acute Lymphoblastic Leukemia (ALL): 80–90% initial response, with about 50–60% long-term remission.
- Diffuse Large B-Cell Lymphoma (DLBCL): 50–60% response rate, with 30–40% achieving complete remission.
- Multiple Myeloma: 70–80% response rate, with about 30–40% achieving complete remission.
- Chronic Lymphocytic Leukemia (CLL): 50–70% response rate, with 30–40% achieving complete remission.
- Solid Tumors: Response rates typically below 30%.
While the success rates of CAR T-cell therapy are promising, it is important to recognize that the treatment is still evolving, and outcomes vary. The therapy continues to improve with better understanding, new products, and ongoing research.
FAQ for car t cell therapy?
Here’s a list of frequently asked questions (FAQ) regarding CAR T-cell therapy, which can help clarify common doubts and provide additional insight into the treatment process.
1. What is CAR T-cell therapy?
CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy) is an innovative cancer treatment that involves genetically modifying a patient's T cells (a type of white blood cell) to better recognize and attack cancer cells. This therapy is primarily used for blood cancers like leukemia and lymphoma.
2. Who is eligible for CAR T-cell therapy?
CAR T-cell therapy is typically recommended for:
- Patients with relapsed or refractory blood cancers, such as acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL), or multiple myeloma.
- Patients who have not responded to traditional therapies like chemotherapy or radiation.
- Age: Both children and adults can be treated, with eligibility determined based on the type of cancer, health status, and prior treatments.
3. How does CAR T-cell therapy work?
The process involves:
- Collection of T cells from the patient’s blood (via leukapheresis).
- Genetic modification of these cells in the lab to add a receptor that targets cancer cells.
- Expansion of the modified T cells.
- Infusion of the engineered T cells back into the patient’s bloodstream.
- The modified T cells then identify and destroy cancer cells.
4. What types of cancer can CAR T-cell therapy treat?
CAR T-cell therapy has shown effectiveness primarily in blood cancers, including:
- Acute Lymphoblastic Leukemia (ALL)
- Diffuse Large B-Cell Lymphoma (DLBCL)
- Chronic Lymphocytic Leukemia (CLL)
- Multiple Myeloma
- Research is ongoing for its use in solid tumors like breast cancer, lung cancer, and glioblastoma, but results so far have been limited.
5. How long does CAR T-cell therapy take?
The entire process can take several weeks:
- T-cell collection (leukapheresis): 1–2 days.
- Genetic modification and expansion: 2–4 weeks.
- Preconditioning chemotherapy: 2–3 days.
- CAR T-cell infusion: 1 day.
6. What are the side effects of CAR T-cell therapy?
CAR T-cell therapy can cause both short-term and long-term side effects:
- Cytokine Release Syndrome (CRS): A common side effect causing fever, low blood pressure, and difficulty breathing.
- Neurotoxicity (ICANS): Confusion, delirium, and, in rare cases, seizures.
- Infections: Due to immune suppression.
- Low blood counts: Can lead to anemia, bleeding, or infection.
- Fatigue, nausea, and pain: Typically related to the preconditioning chemotherapy.
7. What is Cytokine Release Syndrome (CRS)?
CRS is a serious side effect of CAR T-cell therapy caused by the rapid activation and proliferation of CAR T cells, releasing large amounts of cytokines into the bloodstream. Symptoms can range from mild (fever) to severe (difficulty breathing, organ dysfunction). It is treated with medications like tocilizumab and corticosteroids.
8. How is CAR T-cell therapy administered?
After the T cells are modified and expanded in the lab, they are infused back into the patient’s bloodstream through an IV line, similar to a blood transfusion. The process usually takes 30–60 minutes.
9. How successful is CAR T-cell therapy?
Success rates vary depending on the type of cancer:
- Acute Lymphoblastic Leukemia (ALL): High response rate of 80-90%, with 50-60% achieving long-term remission.
- Diffuse Large B-Cell Lymphoma (DLBCL): 50-60% response rate, with 30-40% in complete remission.
- Multiple Myeloma: 70-80% response rate, with 30-40% in complete remission.
- Solid tumors: Early results show lower response rates, generally under 30%.
10. How long do the effects of CAR T-cell therapy last?
- Many patients experience long-term remission, but some may relapse. Monitoring continues for several months or years.
- For certain cancers like DLBCL and ALL, relapses are common within the first 1–2 years.
11. Is CAR T-cell therapy FDA-approved?
Yes, CAR T-cell therapy has received FDA approval for several indications:
- Kymriah (tisagenlecleucel) for B-cell ALL in children and young adults.
- Yescarta (axicabtagene ciloleucel) for DLBCL and other types of B-cell lymphoma.
- Kymriah and Breyanzi (lisocabtagene maraleucel) for adult patients with DLBCL.
- Abecma (idecabtagene vicleucel) for multiple myeloma.
12. What is the cost of CAR T-cell therapy?
In countries like the U.S., CAR T-cell therapy can cost between $373,000–$450,000 for a single treatment cycle. In India, the cost is lower, typically ranging from Rs 30–40 lakh ($35,000–$45,000 USD). Costs vary depending on factors like the healthcare provider and the specific therapy used.
13. Is CAR T-cell therapy covered by insurance?
Some insurance plans may cover CAR T-cell therapy, but it varies by insurer and country. Insurance coverage often depends on the specific cancer, prior treatment history, and the policy’s terms. It's important for patients to verify coverage before undergoing treatment.
14. What is the difference between autologous and allogeneic CAR T-cell therapy?
- Autologous CAR T-cell therapy: The patient's own T cells are used for the treatment.
- Allogeneic CAR T-cell therapy: T cells are collected from a donor (someone other than the patient).
Most CAR T-cell therapies are autologous, as they tend to have fewer complications. However, allogeneic therapies are being explored for patients who cannot undergo autologous treatment.
15. Can CAR T-cell therapy be used for solid tumors?
While CAR T-cell therapy is most successful in treating blood cancers, research is ongoing for its use in solid tumors. Early trials show limited success, and overcoming challenges such as the tumor microenvironment and antigen heterogeneity is a focus of current research.
16. Can I receive CAR T-cell therapy if I’ve had other treatments before?
Yes, CAR T-cell therapy is often used for patients who have relapsed or failed other treatments like chemotherapy, radiation, or stem cell transplants. It is typically considered when other options have not worked.
17. How do I prepare for CAR T-cell therapy?
Preparation involves:
- Medical evaluation: Comprehensive tests to assess overall health and eligibility.
- Leukapheresis: Collection of T cells from your blood.
- Preconditioning chemotherapy: Low-dose chemotherapy to suppress the immune system before CAR T-cell infusion.
18. What happens if CAR T-cell therapy doesn’t work?
If CAR T-cell therapy does not work or the cancer relapses, alternative treatments like other forms of immunotherapy, chemotherapy, or clinical trials may be explored. Researchers are also working on improving CAR T-cell therapies and developing second-line CAR T-cell treatments.
19. Can I travel during or after CAR T-cell therapy?
- During the treatment process, especially the T-cell collection and post-infusion monitoring, patients are usually advised to stay close to the treatment center.
- After therapy, patients can travel but should be cautious about infections, side effects, and regular follow-up care.
20. How long will I need to be monitored after CAR T-cell therapy?
Patients are closely monitored for several weeks to months following treatment. After that, long-term follow-up (6–12 months or longer) is required to monitor for relapse, side effects, and overall health.
These FAQs provide an overview of common concerns, but it’s essential to discuss your individual case with your healthcare provider to get personalized guidance on CAR T-cell therapy.
21. How Much Does CAR T Cell Therapy Cost in India?
The cost of CAR T-cell therapy in India typically ranges between US$50,000 and US$85,000 (approximately Rs 30–40 lakh), which is significantly more affordable compared to countries like the United States, where the cost can exceed $400,000 per treatment cycle.
This cost difference is due to factors such as:
- Lower healthcare costs in India, including labor and facility expenses.
- Regulatory differences, with Indian healthcare providers offering competitive pricing for cutting-edge treatments.
- Availability of local CAR T-cell manufacturing, reducing reliance on international suppliers.
While the treatment is expensive, it remains a more accessible option for many patients in India than in Western countries. Some hospitals in India may also provide financial support programs, and some insurance plans may cover the therapy, depending on the patient's eligibility.
Efforts are being made to reduce the cost of CAR T-cell therapy even further, with the goal of making it more affordable for a larger number of patients.
Top Doctors
Sorry, There is nothing to show!Come back soon.
Top Hospitals
CART CELL THERAPY COST IN INDIA

Duration of Treatment
50000 to 85000 USD
Days of Stay
30 Days
Anesthesia
No
Cost
50000 to 85000 USD
How Much Does CAR T Cell Therapy Cost in India?
Looking for CAR T cell therapy cost in India in different cities like Delhi, Mumbai, Chennai, Kolkata, and Bangalore? Here, we answer the question and explain how to choose the best treatment centers in India for better results. Still, have questions? We have also shortlisted the list of cancer centers in India and doctors based on Hospital accreditations, experience & qualification of surgeons, success rates of procedures, and patient testimonials.
- CART cell therapy cost in India: Starting from US$ 50,000 and US$ 85,000
- Hotel Cost Near Hospital - starting from 18 to 50 USD ( as per hotel services)
- Food Cost - starting from 20 to 30 USD (per day )
- Miscellaneous cost - 20 USD (per day)
To make an appointment, learn more about it, read the below information, or call / WhatsApp/ Viber - our experts to answer at +91 9582708782. Still, have questions?
Cost Variation by City:
- Delhi: Top hospitals like AIIMS and Fortis generally charge in the higher range of the pricing spectrum.
- Mumbai: Leading hospitals such as Tata Memorial and HCG are known for offering high-quality CAR T-cell therapy, with costs typically in the mid to high range.
- Chennai: Hospitals like Apollo Cancer Centre and Sri Ramachandra Medical Centre offer competitive pricing, with costs typically lower than in Delhi or Mumbai.
- Kolkata: Well-regarded hospitals like Tata Medical Center offer affordable treatment options, with pricing around the lower to mid-range.
- Bangalore: Manipal Hospitals and Fortis offer CAR T-cell therapy in the mid to high range.
How to Choose the Best Treatment Centers in India:
When selecting a treatment center for CAR T-cell therapy, consider the following factors:
- Hospital Accreditation: Look for NABH, NABL, or JCI-accredited hospitals, which ensure quality care and standards.
- Experience & Qualifications of Surgeons: Experienced oncologists and hematologists specializing in CAR T-cell therapy are essential for successful outcomes.
- Success Rates: Research success rates for CAR T-cell therapy, especially for the specific cancer type you're being treated for.
- Patient Testimonials: Look for positive patient reviews and testimonials, particularly from those who have undergone CAR T-cell therapy at the hospital.
- Post-Treatment Care: Consider the hospital’s post-treatment care protocols, as CAR T-cell therapy can require extensive follow-up.
Contact Information:
If you’re interested in scheduling a consultation, you can contact experts or receive more detailed information by calling or messaging:
- Phone/WhatsApp/Viber: +91 9582708782
Summary:
The cost of CAR T-cell therapy in India is considerably lower than in many Western countries, with a starting price of around US$ 50,000, depending on the city and hospital. In addition to the therapy itself, other expenses such as accommodation, food, and miscellaneous costs should be factored in. By choosing an accredited hospital with experienced doctors, you can improve your chances of successful treatment.
What is CAR T Cell Therapy?
CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy) is an advanced form of immunotherapy used primarily to treat certain types of cancer, particularly blood cancers such as leukemia, lymphoma, and multiple myeloma. It involves reprogramming a patient’s own immune cells to target and kill cancer cells.
CAR T-cell therapy comes in various types, categorized based on the specific antigens they target or the technologies used to improve their effectiveness. Here are the primary types of CAR T-cell therapies:
1. Antigen-Specific CAR T-Cell Therapies
These therapies are classified by the specific protein (antigen) they target on cancer cells:
-
CD19 CAR T-cell Therapy:
- Targets CD19, a protein commonly found on B cells.
- Effective for:
- Acute Lymphoblastic Leukemia (ALL)
- Diffuse Large B-Cell Lymphoma (DLBCL)
- Mantle Cell Lymphoma
- Examples: Kymriah (tisagenlecleucel), Yescarta (axicabtagene ciloleucel), Breyanzi (lisocabtagene maraleucel).
-
BCMA CAR T-cell Therapy:
- Targets BCMA (B-cell maturation antigen), found on plasma cells.
- Effective for:
- Multiple Myeloma
- Examples: Abecma (idecabtagene vicleucel), Carvykti (ciltacabtagene autoleucel).
-
CD22 CAR T-cell Therapy:
- Targets CD22, another protein found on B cells.
- Primarily used in research or for cases where CD19 therapy is less effective.
-
Dual-Antigen CAR T-cell Therapy:
- Targets two antigens (e.g., CD19 and CD22) simultaneously.
- Designed to overcome resistance by ensuring cancer cells cannot evade therapy by losing a single antigen.
2. Generational CAR T-Cell Therapies
CAR T-cell therapy has evolved over time, with improvements in design:
-
First-Generation CARs:
- Basic design with limited effectiveness.
- Rarely used today.
-
Second-Generation CARs:
- Incorporate one costimulatory domain (e.g., CD28 or 4-1BB) to enhance T-cell activation and survival.
- Most FDA-approved therapies fall into this category.
-
Third-Generation CARs:
- Combine multiple costimulatory domains for greater potency.
- Primarily used in research settings.
-
Fourth-Generation CARs (TRUCKs):
- "T-cells Redirected for Universal Cytokine Killing."
- Engineered to deliver cytokines to enhance the immune response at the tumor site.
-
Fifth-Generation CARs:
- Include intracellular signaling pathways to improve efficacy and manage resistance.
3. Universal or Off-the-Shelf CAR T-Cell Therapies
- Allogeneic CAR T-Cell Therapy:
- Uses T cells from a healthy donor rather than the patient’s own cells.
- Reduces wait times and makes therapy more accessible.
- Example: Research in therapies like AlloCAR T.
4. Tumor Microenvironment-Targeting CAR T-Cell Therapies
- Designed to address barriers in the tumor environment, such as immune suppression or poor T-cell infiltration.
- Still largely in experimental stages.
5. Solid Tumor CAR T-Cell Therapies
- Target antigens like HER2, EGFR, or GD2, which are expressed in solid tumors.
- Development is more challenging due to antigen heterogeneity and the immunosuppressive nature of solid tumors.
These types of CAR T-cell therapies represent a rapidly advancing field, with ongoing research aimed at making them safer, more effective, and applicable to a wider range of cancers.
Is CAR-T cell therapy Available in India?
Yes, CAR T-cell therapy is available in India. In April 2024, India celebrated the launch of its first homegrown CAR T-cell therapy, NexCAR19™ (actalycabtagene autoleucel), developed through a collaboration between the Indian Institute of Technology, Bombay, Tata Memorial Hospital, and ImmunoACT.
This therapy has been approved for the treatment of relapsed or refractory B-cell lymphomas and leukemia in patients aged 15 years and above.
car-t cell therapy hospitals in India : Several leading hospitals in India now offer CAR T-cell therapy:
-
Apollo Cancer Centres: As the first private hospital group in India to launch a CAR T-cell program, Apollo provides access to NexCAR19™ for treating B-cell lymphomas and B-acute lymphoblastic leukemia.
-
Max Healthcare: Located in Delhi, Max Healthcare offers CAR T-cell therapy for various blood cancers, including acute lymphoblastic leukemia and diffuse large B-cell lymphoma.
-
Artemis Hospital: Gurugram, India, offers advanced cancer treatments, including CAR T-cell therapy. This therapy involves modifying a patient's T-cells to enhance their ability to combat cancer. Artemis Hospitals has successfully treated patients with aggressive forms of lymphoma using CAR T-cell therapy. For instance, Ms. Linda Becht from the Netherlands underwent successful treatment for an aggressive form of lymphoma at Artemis Hospitals.
For more detailed information or to determine eligibility for CAR T-cell therapy at Artemis Hospitals, it is advisable to contact their oncology department directly.
The cost of CAR T-cell therapy in India ranges between US$50,000 and US$85,000, which is significantly more affordable compared to prices in countries like the United States, where costs can exceed $400,000.
This development positions India as a key player in providing advanced and cost-effective CAR T-cell therapies, making it accessible to a broader patient population.
n India, the cost of CAR T-cell therapy ranges from approximately ₹30–40 lakh (3–4 million INR) as of April 2024. This cost is significantly lower compared to the treatment in other countries like the United States, where it can exceed $400,000.
Efforts to Reduce Costs
- There is a concerted effort to further reduce the cost in India to ₹10–20 lakh (1–2 million INR) in the future.
- The lower cost in India is partly due to more affordable labor and localized manufacturing.
Insurance Coverage
- Some insurance companies in India may cover CAR T-cell therapy for eligible patients, but not all policies include this treatment. It is advisable to check with individual insurers.
NexCAR19
- NexCAR19 is a CAR T-cell therapy developed by ImmunoACT, a collaboration involving the Indian Institute of Technology Bombay, Tata Memorial Hospital, and others.
- It is recognized as one of the most affordable CAR T-cell therapies globally.
- ImmunoACT manufactures CAR T cells at its facility in Mumbai, ensuring localized production and lower costs.
Why is CAR T-cell Therapy Cheaper in India?
- Localized Manufacturing: Facilities in Mumbai produce the therapy domestically, avoiding international transportation and import costs.
- Lower Labor Costs: India's healthcare and research sectors benefit from lower operational expenses.
- Government and Research Support: Collaborative efforts with institutions like IIT Bombay and Tata Memorial Hospital help subsidize development costs.
Conclusion
India is emerging as a global hub for CAR T-cell therapy, offering advanced treatment options at significantly reduced costs while maintaining high standards of care.
symptoms
Who is a candidate for CAR T-cell therapy?
Candidates for CAR T-cell therapy are typically patients with certain types of cancers who meet specific criteria. This therapy is most often used for people who have not responded to standard treatments or whose cancer has returned after remission. Here's an overview of who might be eligible:
1. Types of Cancers Treated
CAR T-cell therapy is primarily approved for the following cancers:
- Blood Cancers:
- Acute Lymphoblastic Leukemia (ALL):
- Particularly in children and young adults.
- Candidates often have relapsed or refractory (R/R) disease.
- B-Cell Non-Hodgkin Lymphomas:
- Diffuse Large B-Cell Lymphoma (DLBCL)
- Mantle Cell Lymphoma (MCL)
- Follicular Lymphoma (FL)
- Multiple Myeloma:
- Targeting BCMA antigen in relapsed/refractory cases.
- Acute Lymphoblastic Leukemia (ALL):
- Other Emerging Indications:
- Research trials are exploring CAR T-cell therapy for solid tumors (e.g., glioblastoma, lung cancer) and additional blood cancers.
2. Criteria for Eligibility
Patients must generally meet these conditions to qualify:
- Relapsed or Refractory Cancer:
- Cancer that has not responded to two or more lines of conventional treatment (e.g., chemotherapy, radiation, targeted therapy).
- Adequate Organ Function:
- Sufficient heart, liver, kidney, and lung function to handle the therapy and potential side effects.
- Good Performance Status:
- Patients should have a reasonable level of physical fitness (e.g., ECOG performance status of 0-2).
- No Active Infections:
- To reduce the risk of complications during therapy.
- Appropriate Age:
- Age limits may vary based on cancer type, clinical trial criteria, or specific therapy protocols (e.g., some therapies are approved for pediatric patients with ALL).
3. Exclusions
Patients may not qualify if they have:
- Active or uncontrolled infections.
- Severe organ dysfunction or poor overall health.
- Autoimmune diseases that could be exacerbated by therapy.
- Recent history of other aggressive cancers.
4. Special Considerations
- Clinical Trials:
- Patients with cancers not yet approved for CAR T-cell therapy (e.g., solid tumors) may participate in clinical trials if they meet the inclusion criteria.
- Insurance and Cost:
- Availability and eligibility can also depend on financial or insurance coverage for the treatment.
5. Personalized Evaluation
Each candidate is thoroughly evaluated by an oncology and immunotherapy team to ensure suitability, balancing potential benefits with risks. This includes diagnostic tests, imaging, and a review of medical history.
risk factors
What is the Risk for CART cell therapy?
CAR T-cell therapy, while highly effective for certain cancers, carries significant risks due to its potent immune-modulating effects. These risks stem from the therapy's mechanism of action, where engineered T cells aggressively attack cancer cells but can also affect normal tissues or trigger systemic immune responses. Below are the primary risks and side effects associated with CAR T-cell therapy:
1. Cytokine Release Syndrome (CRS)
- What It Is: A systemic inflammatory response caused by the rapid activation and proliferation of CAR T cells, leading to the release of large amounts of cytokines.
- Symptoms:
- High fever
- Low blood pressure
- Difficulty breathing
- Rapid heart rate
- Organ dysfunction (e.g., kidneys, liver)
- Severity:
- Ranges from mild to life-threatening (grade 1–4).
- Management:
- Anti-inflammatory drugs like tocilizumab (IL-6 receptor antagonist) or corticosteroids.
2. Neurological Toxicity (Immune Effector Cell-Associated Neurotoxicity Syndrome, ICANS)
- What It Is: Neurotoxicity caused by the immune response, often accompanying or following CRS.
- Symptoms:
- Confusion or delirium
- Seizures
- Difficulty speaking or slurred speech
- Loss of consciousness
- Management:
- Supportive care and, in severe cases, corticosteroids.
3. Prolonged Low Blood Cell Counts (Cytopenias)
- What It Is: Suppression of normal blood cell production in the bone marrow.
- Risks:
- Anemia (low red blood cells)
- Increased risk of infections (low white blood cells)
- Bleeding tendencies (low platelets)
- Management:
- Blood transfusions or growth factors (e.g., G-CSF).
4. Infections
- What It Is: Increased susceptibility to infections due to immune suppression.
- Causes:
- Initial lymphodepleting chemotherapy before CAR T infusion.
- Prolonged depletion of B cells (hypogammaglobulinemia).
- Management:
- Antibiotics, antivirals, or antifungals.
- Immunoglobulin replacement therapy.
5. Tumor Lysis Syndrome (TLS)
- What It Is: A condition caused by the rapid breakdown of cancer cells, releasing their contents into the bloodstream.
- Risks:
- Kidney damage
- Electrolyte imbalances (e.g., high potassium, uric acid, and phosphate levels)
- Management:
- Hydration, medications like allopurinol, and monitoring electrolyte levels.
6. On-Target, Off-Tumor Toxicity
- What It Is: Damage to normal cells expressing the same antigen as cancer cells.
- Risks:
- Varies depending on the targeted antigen (e.g., CD19 can also affect normal B cells).
7. Graft Versus Host Disease (GVHD) (in allogeneic CAR T-cell therapies):
- What It Is: Immune reaction where donor T cells attack the recipient's tissues.
- Management:
- Immunosuppressive therapy.
8. Long-Term Risks
- Relapse or Resistance: Cancer may return or become resistant to CAR T cells.
- Secondary Cancers: Rare cases of therapy-induced malignancies.
- Long-Term Immunosuppression: Persistent depletion of normal immune cells.
9. Psychological and Emotional Impact
- The intensive nature of treatment and side effects can lead to emotional and mental health challenges, including anxiety and depression.
Mitigation Strategies
- Close Monitoring: Patients are monitored closely in specialized centers for early detection and management of side effects.
- Multidisciplinary Care: A team of oncologists, immunologists, and other specialists ensures comprehensive care.
- Patient Selection: Only patients with adequate organ function and overall health are considered for therapy.
While the risks of CAR T-cell therapy are substantial, advancements in management and early detection have significantly improved patient outcomes.
preparation
What will be the preparation before car t cell therapy?
Preparation for CAR T-cell therapy is a detailed process designed to maximize its effectiveness while minimizing potential risks. Patients undergo several steps, including evaluations, pre-treatment procedures, and supportive care planning. Here's what preparation typically involves:
1. Initial Evaluation
- Medical History and Physical Exam:
- A thorough review of the patient’s medical history, current medications, and overall health.
- Cancer Assessment:
- Tests to confirm the type, stage, and status of the cancer.
- Imaging studies (CT, PET, or MRI scans).
- Lab Tests:
- Blood work to evaluate organ function (kidney, liver, heart), infection risk, and overall health.
- Bone Marrow Biopsy:
- For patients with blood cancers, to assess the extent of disease.
2. Eligibility Assessment
- Performance Status:
- Determination of the patient’s physical ability to tolerate the therapy (e.g., ECOG or Karnofsky Performance Status).
- Organ Function:
- Heart: Echocardiogram or MUGA scan.
- Lungs: Pulmonary function tests.
- Liver and Kidney: Comprehensive metabolic panel.
- Infection Screening:
- Testing for infections such as HIV, hepatitis, and tuberculosis to ensure safety during therapy.
3. Collection of T Cells (Leukapheresis)
- Leukapheresis Procedure:
- T cells are collected from the patient’s blood using a machine that separates and collects white blood cells.
- The process takes several hours and may require multiple sessions.
- Post-Collection Care:
- Patients are monitored for side effects, such as fatigue or low calcium levels.
4. Waiting Period (Manufacturing Phase)
- CAR T-Cell Production:
- The collected T cells are genetically engineered in a laboratory to produce chimeric antigen receptors (CARs) that target cancer cells.
- This process typically takes 2–4 weeks.
- Bridging Therapy (if needed):
- If the cancer is progressing rapidly, patients may receive interim treatments (e.g., chemotherapy, radiation) to control disease until the CAR T cells are ready.
5. Preconditioning Chemotherapy (Lymphodepletion)
- Purpose:
- Low-dose chemotherapy is given to suppress the immune system, creating space for the infused CAR T cells to expand and work effectively.
- Timing:
- Administered a few days before the CAR T-cell infusion.
- Common Drugs Used:
- Cyclophosphamide and fludarabine.
6. Hospital Admission
- Hospitalization:
- Many patients are admitted to a specialized cancer center for monitoring during and after the CAR T-cell infusion.
- In some cases, outpatient therapy is possible, but close proximity to the hospital is required.
7. Supportive Care Planning
- Infection Prevention:
- Prophylactic antibiotics, antivirals, or antifungals may be prescribed.
- Emergency Plan:
- Detailed protocols for managing potential side effects like cytokine release syndrome (CRS) or neurotoxicity.
- Caregiver Support:
- Patients need a caregiver or family member to assist during recovery.
- Psychological Preparation:
- Counseling or support groups may help address emotional and mental health concerns.
8. Consent and Education
- Informed Consent:
- Patients are educated about the risks, benefits, and potential outcomes of CAR T-cell therapy.
- Educational Sessions:
- Detailed explanations about the therapy process, side effects, and post-treatment expectations.
9. Nutritional and Physical Preparation
- Healthy Diet:
- Maintaining good nutrition to support immune function and recovery.
- Physical Activity:
- Gentle exercise to improve physical fitness before treatment.
Summary Timeline
- Weeks 1–2: Initial evaluation and eligibility determination.
- Week 3: Leukapheresis (T-cell collection).
- Weeks 4–8: CAR T-cell production; bridging therapy if needed.
- Final Week: Preconditioning chemotherapy and admission for infusion.
This detailed preparation helps ensure patients are in the best possible condition to receive CAR T-cell therapy and manage its potential side effects effectively.
procedure
what will be the procedure CART cell therapy?
The procedure for CAR T-cell therapy involves several steps, from the collection of T cells to their genetic modification, infusion, and post-treatment monitoring. Here's an overview of the process:
1. T-Cell Collection (Leukapheresis)
- Purpose: To collect T cells from the patient's blood.
- Procedure:
- The patient is connected to a machine via an IV line or central catheter.
- Blood is drawn, and the machine separates white blood cells (including T cells) from the rest of the blood.
- The remaining blood components are returned to the patient.
- Duration: Takes about 2–4 hours and is generally done in an outpatient setting.
2. Genetic Modification (CAR T-Cell Manufacturing)
- Purpose: To engineer the patient’s T cells to better recognize and attack cancer cells.
- Procedure:
- The collected T cells are sent to a specialized laboratory.
- The cells are genetically modified using a viral vector to add a chimeric antigen receptor (CAR), enabling them to target a specific protein (antigen) on cancer cells.
- The modified T cells are then grown and expanded in the lab to produce millions of CAR T cells.
- Duration: This step takes 2–4 weeks.
3. Preconditioning Chemotherapy (Lymphodepletion)
- Purpose: To suppress the patient’s immune system and create a favorable environment for the infused CAR T cells to work effectively.
- Procedure:
- Low-dose chemotherapy is administered over 2–3 days before the CAR T-cell infusion.
- Common drugs: Cyclophosphamide and Fludarabine.
- Monitoring: Patients are closely monitored for side effects like fatigue or low blood counts.
4. CAR T-Cell Infusion
- Purpose: To reintroduce the engineered CAR T cells into the patient’s body to attack cancer cells.
- Procedure:
- CAR T cells are thawed and infused intravenously, similar to a blood transfusion.
- The infusion usually takes 30–60 minutes but may vary depending on the dose.
- Hospital Setting:
- This step is performed in a specialized cancer center.
- Patients are monitored closely during the infusion for immediate side effects.
5. Post-Infusion Monitoring
- Purpose: To manage potential side effects and monitor the effectiveness of the therapy.
- Steps:
- In-Hospital Monitoring:
- Patients are typically observed for 7–14 days for acute complications like Cytokine Release Syndrome (CRS) or neurotoxicity.
- Post-Discharge Monitoring:
- Regular follow-ups for 4–8 weeks to monitor recovery and response to therapy.
- Long-Term Monitoring:
- Periodic check-ups for up to a year or more to assess the treatment’s success and detect any delayed side effects.
- In-Hospital Monitoring:
6. Management of Side Effects
- Cytokine Release Syndrome (CRS):
- Symptoms: Fever, low blood pressure, and breathing difficulties.
- Treated with medications like tocilizumab and corticosteroids.
- Neurotoxicity (ICANS):
- Symptoms: Confusion, seizures, or speech issues.
- Treated with supportive care and corticosteroids.
7. Follow-Up and Support
- Efficacy Evaluation:
- Imaging scans (CT, PET) and blood tests are performed to determine the cancer’s response to therapy.
- Rehabilitation:
- Physical and emotional support for recovery.
- Immunological Monitoring:
- Monitoring for prolonged immune suppression or relapse.
- Secondary Treatments:
- Additional interventions may be required if the cancer doesn’t respond fully.
Summary Timeline
- T-Cell Collection: 1 day.
- T-Cell Engineering: 2–4 weeks.
- Preconditioning Chemotherapy: 2–3 days.
- CAR T-Cell Infusion: 1 day.
- Post-Treatment Monitoring: Weeks to months.
This structured approach ensures safety and maximizes the effectiveness of CAR T-cell therapy for patients with eligible cancers.
post procedure
What will be the post-care for CAR T Cell therapy?
Post-care for CAR T-cell therapy is critical to monitor for side effects, evaluate the therapy’s effectiveness, and ensure a smooth recovery. The post-care period involves close observation, regular follow-ups, and supportive care to address potential complications and long-term effects. Here's a detailed breakdown of post-care:
1. Immediate Post-Treatment Monitoring
- Hospital Stay:
- Most patients are monitored in the hospital for 1–2 weeks after the CAR T-cell infusion.
- Vital signs and symptoms are observed for immediate side effects, especially Cytokine Release Syndrome (CRS) and neurotoxicity (ICANS).
- Treatments like tocilizumab (IL-6 inhibitor) or corticosteroids may be administered as needed.
- Isolation Precautions:
- To prevent infections due to temporary immune suppression, patients are often kept in sterile environments.
2. Short-Term Care (First 4–8 Weeks)
- Frequent Medical Visits:
- Regular follow-ups with the oncology team to monitor recovery, manage side effects, and evaluate the therapy’s effectiveness.
- Blood tests to assess organ function, immune cell counts, and signs of relapse.
- Infection Prevention:
- Preventive antibiotics, antivirals, or antifungals may be prescribed.
- Vaccination schedules are deferred or carefully planned to avoid live vaccines during immune suppression.
- Symptom Management:
- Addressing fatigue, nausea, pain, or other lingering effects from chemotherapy or the infusion process.
- Emotional and Psychological Support:
- Counseling or support groups to help patients cope with the emotional challenges of cancer treatment.
3. Long-Term Follow-Up (Months to Years)
- Efficacy Evaluation:
- Imaging (CT, PET scans) and bone marrow biopsies to confirm cancer remission or response to treatment.
- Regular blood tests to monitor for cancer recurrence.
- Monitoring for Late-Onset Side Effects:
- Persistent cytopenias (low blood cell counts).
- Delayed neurological symptoms.
- Prolonged immune suppression leading to infections.
- B-Cell Aplasia (if CAR T cells target CD19):
- Patients may need immunoglobulin replacement therapy to prevent infections.
4. Rehabilitation and Quality of Life
- Physical Rehabilitation:
- Physical therapy to regain strength and stamina after prolonged hospitalization or treatment.
- Nutritional Support:
- A balanced diet to support recovery and immune function.
- Mental Health Support:
- Addressing anxiety, depression, or post-traumatic stress related to cancer and its treatment.
5. Secondary Treatments or Maintenance
- Treatment for Residual Cancer:
- In cases where cancer persists, additional therapies may be considered (e.g., radiation, chemotherapy, or clinical trials).
- Management of Relapse:
- Patients are monitored closely for signs of cancer recurrence and may undergo another round of CAR T-cell therapy or alternative treatments.
6. Education for Patients and Caregivers
- Symptom Awareness:
- Patients and caregivers are trained to recognize early signs of complications, such as fever, confusion, or shortness of breath.
- Emergency Plan:
- Clear instructions on when and how to seek medical help in case of severe symptoms.
- Lifestyle Adjustments:
- Avoiding exposure to infections (e.g., crowded places).
- Modifications to work or daily activities based on recovery progress.
7. Re-Vaccination
- Vaccination Schedule:
- Following immune recovery, patients may need re-vaccination, particularly for diseases like measles, mumps, rubella, and pneumococcus.
Potential Long-Term Risks to Monitor
- Cancer Relapse or Resistance:
- Periodic testing to check for recurrence or resistance to CAR T cells.
- Secondary Malignancies:
- Rare cases of treatment-related cancers.
- Chronic Graft-Versus-Host Disease (in allogeneic CAR T therapy):
- Long-term immunosuppressive management if this occurs.
8. Financial and Logistical Support
- Insurance and Cost Management:
- Assistance with claims or financial aid for follow-up care and medications.
- Travel Logistics:
- Patients treated far from home may need to stay near the treatment center for follow-ups during the first few months.
Summary
Post-care for CAR T-cell therapy is a long-term process aimed at:
- Managing acute and late-onset side effects.
- Monitoring the therapy’s success in treating cancer.
- Providing physical, emotional, and logistical support for recovery.
Patients and caregivers play an essential role in ensuring adherence to follow-up schedules and promptly reporting any concerning symptoms.
success rate
What will be the success rate for CAR T-cell therapy?
The success rate of CAR T-cell therapy varies depending on several factors, including the type of cancer being treated, the patient’s overall health, the specific CAR T-cell product used, and how well the patient responds to the therapy. However, CAR T-cell therapy has shown impressive results, particularly for certain blood cancers. Below are general success rates and factors that influence outcomes:
Success Rates in Blood Cancers (Most Common Indications)
-
Acute Lymphoblastic Leukemia (ALL)
- Children and Young Adults: For B-cell ALL, especially in pediatric and young adult patients, CAR T-cell therapy has shown a high response rate.
- Complete Remission: Around 80–90% of children and young adults with relapsed or refractory B-ALL achieve complete remission (CR) after CAR T-cell therapy.
- Long-Term Remission: However, about 50–60% of these patients may experience long-term remission, with the remainder relapsing or experiencing recurrence within 1–2 years.
- Children and Young Adults: For B-cell ALL, especially in pediatric and young adult patients, CAR T-cell therapy has shown a high response rate.
-
Diffuse Large B-Cell Lymphoma (DLBCL)
- In patients with relapsed or refractory DLBCL, CAR T-cell therapy has demonstrated a response rate of approximately:
- Overall Response Rate: Around 50–60% of patients show a positive response.
- Complete Remission: Roughly 30–40% of patients achieve complete remission.
- However, outcomes can be affected by factors such as age, prior treatments, and the specific subtype of lymphoma.
- In patients with relapsed or refractory DLBCL, CAR T-cell therapy has demonstrated a response rate of approximately:
-
Multiple Myeloma
- CAR T-cell therapy targeting BCMA (B-cell maturation antigen) in multiple myeloma has shown promising results:
- Response Rate: Around 70–80% of patients with relapsed/refractory multiple myeloma experience a positive response.
- Complete Remission: Approximately 30–40% may achieve complete remission, though relapses are common.
- CAR T-cell therapy targeting BCMA (B-cell maturation antigen) in multiple myeloma has shown promising results:
-
Chronic Lymphocytic Leukemia (CLL)
- For CLL, especially when it relapses or becomes resistant to other treatments, CAR T-cell therapy has a response rate of 50–70%, with a complete remission rate of around 30–40%.
Success Rates in Solid Tumors
- Solid tumors (e.g., lung cancer, breast cancer, glioblastoma) are more challenging to treat with CAR T-cell therapy.
- Response Rates: Early clinical trials have shown limited success, with response rates generally below 30%.
- Challenges: Solid tumors present difficulties like immune suppression by the tumor microenvironment, antigen heterogeneity, and physical barriers (e.g., the blood-brain barrier).
- Ongoing research is focused on improving CAR T-cell effectiveness for solid tumors, and success rates may improve as new therapies are developed.
Factors Affecting Success Rates
- Type of Cancer: Blood cancers like leukemia and lymphoma tend to have better outcomes with CAR T-cell therapy than solid tumors.
- Disease Stage:
- Patients with early-stage cancer or those who achieve a deep remission before CAR T-cell therapy generally have better outcomes.
- Relapsed or refractory cases may have lower success rates, especially if the cancer has developed resistance to previous treatments.
- Patient Characteristics:
- Age: Younger patients, especially children and young adults, tend to have higher response rates.
- Health and Performance Status: Patients in good overall health, with well-functioning organs and good performance status, are more likely to have a positive outcome.
- CAR T-Cell Product: Different CAR T-cell products may vary in efficacy, with newer therapies and customized treatments showing higher success in some cases.
- Manufacturing Success: The quality of T-cell manufacturing (e.g., expansion and persistence of the engineered cells) can impact the success of the therapy.
- Side Effects: Severe side effects (e.g., cytokine release syndrome, neurotoxicity) can negatively impact treatment success, either temporarily or permanently.
Relapse and Long-Term Outcomes
-
Relapse:
- Even after initial remission, some patients may relapse within months or years of CAR T-cell therapy. The relapse rate is generally higher in patients with more aggressive or refractory cancers.
- New treatments or second-line CAR T-cell therapies are being explored for these cases.
-
Long-Term Success:
- Long-term survival rates have improved, especially for patients with blood cancers like ALL, DLBCL, and multiple myeloma, but the success rate is not guaranteed for every patient.
Overall Summary of Success Rates
- Acute Lymphoblastic Leukemia (ALL): 80–90% initial response, with about 50–60% long-term remission.
- Diffuse Large B-Cell Lymphoma (DLBCL): 50–60% response rate, with 30–40% achieving complete remission.
- Multiple Myeloma: 70–80% response rate, with about 30–40% achieving complete remission.
- Chronic Lymphocytic Leukemia (CLL): 50–70% response rate, with 30–40% achieving complete remission.
- Solid Tumors: Response rates typically below 30%.
While the success rates of CAR T-cell therapy are promising, it is important to recognize that the treatment is still evolving, and outcomes vary. The therapy continues to improve with better understanding, new products, and ongoing research.
faqs from doctor
FAQ for car t cell therapy?
Here’s a list of frequently asked questions (FAQ) regarding CAR T-cell therapy, which can help clarify common doubts and provide additional insight into the treatment process.
1. What is CAR T-cell therapy?
CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy) is an innovative cancer treatment that involves genetically modifying a patient's T cells (a type of white blood cell) to better recognize and attack cancer cells. This therapy is primarily used for blood cancers like leukemia and lymphoma.
2. Who is eligible for CAR T-cell therapy?
CAR T-cell therapy is typically recommended for:
- Patients with relapsed or refractory blood cancers, such as acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL), or multiple myeloma.
- Patients who have not responded to traditional therapies like chemotherapy or radiation.
- Age: Both children and adults can be treated, with eligibility determined based on the type of cancer, health status, and prior treatments.
3. How does CAR T-cell therapy work?
The process involves:
- Collection of T cells from the patient’s blood (via leukapheresis).
- Genetic modification of these cells in the lab to add a receptor that targets cancer cells.
- Expansion of the modified T cells.
- Infusion of the engineered T cells back into the patient’s bloodstream.
- The modified T cells then identify and destroy cancer cells.
4. What types of cancer can CAR T-cell therapy treat?
CAR T-cell therapy has shown effectiveness primarily in blood cancers, including:
- Acute Lymphoblastic Leukemia (ALL)
- Diffuse Large B-Cell Lymphoma (DLBCL)
- Chronic Lymphocytic Leukemia (CLL)
- Multiple Myeloma
- Research is ongoing for its use in solid tumors like breast cancer, lung cancer, and glioblastoma, but results so far have been limited.
5. How long does CAR T-cell therapy take?
The entire process can take several weeks:
- T-cell collection (leukapheresis): 1–2 days.
- Genetic modification and expansion: 2–4 weeks.
- Preconditioning chemotherapy: 2–3 days.
- CAR T-cell infusion: 1 day.
6. What are the side effects of CAR T-cell therapy?
CAR T-cell therapy can cause both short-term and long-term side effects:
- Cytokine Release Syndrome (CRS): A common side effect causing fever, low blood pressure, and difficulty breathing.
- Neurotoxicity (ICANS): Confusion, delirium, and, in rare cases, seizures.
- Infections: Due to immune suppression.
- Low blood counts: Can lead to anemia, bleeding, or infection.
- Fatigue, nausea, and pain: Typically related to the preconditioning chemotherapy.
7. What is Cytokine Release Syndrome (CRS)?
CRS is a serious side effect of CAR T-cell therapy caused by the rapid activation and proliferation of CAR T cells, releasing large amounts of cytokines into the bloodstream. Symptoms can range from mild (fever) to severe (difficulty breathing, organ dysfunction). It is treated with medications like tocilizumab and corticosteroids.
8. How is CAR T-cell therapy administered?
After the T cells are modified and expanded in the lab, they are infused back into the patient’s bloodstream through an IV line, similar to a blood transfusion. The process usually takes 30–60 minutes.
9. How successful is CAR T-cell therapy?
Success rates vary depending on the type of cancer:
- Acute Lymphoblastic Leukemia (ALL): High response rate of 80-90%, with 50-60% achieving long-term remission.
- Diffuse Large B-Cell Lymphoma (DLBCL): 50-60% response rate, with 30-40% in complete remission.
- Multiple Myeloma: 70-80% response rate, with 30-40% in complete remission.
- Solid tumors: Early results show lower response rates, generally under 30%.
10. How long do the effects of CAR T-cell therapy last?
- Many patients experience long-term remission, but some may relapse. Monitoring continues for several months or years.
- For certain cancers like DLBCL and ALL, relapses are common within the first 1–2 years.
11. Is CAR T-cell therapy FDA-approved?
Yes, CAR T-cell therapy has received FDA approval for several indications:
- Kymriah (tisagenlecleucel) for B-cell ALL in children and young adults.
- Yescarta (axicabtagene ciloleucel) for DLBCL and other types of B-cell lymphoma.
- Kymriah and Breyanzi (lisocabtagene maraleucel) for adult patients with DLBCL.
- Abecma (idecabtagene vicleucel) for multiple myeloma.
12. What is the cost of CAR T-cell therapy?
In countries like the U.S., CAR T-cell therapy can cost between $373,000–$450,000 for a single treatment cycle. In India, the cost is lower, typically ranging from Rs 30–40 lakh ($35,000–$45,000 USD). Costs vary depending on factors like the healthcare provider and the specific therapy used.
13. Is CAR T-cell therapy covered by insurance?
Some insurance plans may cover CAR T-cell therapy, but it varies by insurer and country. Insurance coverage often depends on the specific cancer, prior treatment history, and the policy’s terms. It's important for patients to verify coverage before undergoing treatment.
14. What is the difference between autologous and allogeneic CAR T-cell therapy?
- Autologous CAR T-cell therapy: The patient's own T cells are used for the treatment.
- Allogeneic CAR T-cell therapy: T cells are collected from a donor (someone other than the patient).
Most CAR T-cell therapies are autologous, as they tend to have fewer complications. However, allogeneic therapies are being explored for patients who cannot undergo autologous treatment.
15. Can CAR T-cell therapy be used for solid tumors?
While CAR T-cell therapy is most successful in treating blood cancers, research is ongoing for its use in solid tumors. Early trials show limited success, and overcoming challenges such as the tumor microenvironment and antigen heterogeneity is a focus of current research.
16. Can I receive CAR T-cell therapy if I’ve had other treatments before?
Yes, CAR T-cell therapy is often used for patients who have relapsed or failed other treatments like chemotherapy, radiation, or stem cell transplants. It is typically considered when other options have not worked.
17. How do I prepare for CAR T-cell therapy?
Preparation involves:
- Medical evaluation: Comprehensive tests to assess overall health and eligibility.
- Leukapheresis: Collection of T cells from your blood.
- Preconditioning chemotherapy: Low-dose chemotherapy to suppress the immune system before CAR T-cell infusion.
18. What happens if CAR T-cell therapy doesn’t work?
If CAR T-cell therapy does not work or the cancer relapses, alternative treatments like other forms of immunotherapy, chemotherapy, or clinical trials may be explored. Researchers are also working on improving CAR T-cell therapies and developing second-line CAR T-cell treatments.
19. Can I travel during or after CAR T-cell therapy?
- During the treatment process, especially the T-cell collection and post-infusion monitoring, patients are usually advised to stay close to the treatment center.
- After therapy, patients can travel but should be cautious about infections, side effects, and regular follow-up care.
20. How long will I need to be monitored after CAR T-cell therapy?
Patients are closely monitored for several weeks to months following treatment. After that, long-term follow-up (6–12 months or longer) is required to monitor for relapse, side effects, and overall health.
These FAQs provide an overview of common concerns, but it’s essential to discuss your individual case with your healthcare provider to get personalized guidance on CAR T-cell therapy.
21. How Much Does CAR T Cell Therapy Cost in India?
The cost of CAR T-cell therapy in India typically ranges between US$50,000 and US$85,000 (approximately Rs 30–40 lakh), which is significantly more affordable compared to countries like the United States, where the cost can exceed $400,000 per treatment cycle.
This cost difference is due to factors such as:
- Lower healthcare costs in India, including labor and facility expenses.
- Regulatory differences, with Indian healthcare providers offering competitive pricing for cutting-edge treatments.
- Availability of local CAR T-cell manufacturing, reducing reliance on international suppliers.
While the treatment is expensive, it remains a more accessible option for many patients in India than in Western countries. Some hospitals in India may also provide financial support programs, and some insurance plans may cover the therapy, depending on the patient's eligibility.
Efforts are being made to reduce the cost of CAR T-cell therapy even further, with the goal of making it more affordable for a larger number of patients.
Top Doctors
Sorry, There is nothing to show!Come back soon.




















